How Decentralized Clinical Trials and Real-World Evidence are Redefining Clinical Trials
Discover how Decentralized Clinical Trials & Real-worldEvidence are transforming patient care & research efficiency at the upcoming MarketsandMarkets Conference
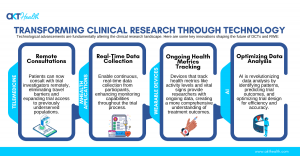
With the traditional model of site-based clinical trials often facing challenges like limited patient recruitment and high dropout rates, DCTs are offering a solution that removes geographical barriers and engages a broader, more diverse population. By leveraging digital technologies—such as telemedicine, wearable devices, and mobile health (mHealth) applications—patients can now participate in trials remotely, allowing for faster recruitment, improved retention, and a more accurate representation of real-world populations.
The integration of these technologies into clinical research not only enhances patient experience but also improves data quality and accelerates the drug development timeline. Real-time data collection and virtual monitoring from patients' homes contribute to more robust datasets, providing pharmaceutical companies with the insights needed to bring life-saving treatments to market more quickly.
Moreover, Real-World Evidence (RWE) is becoming indispensable in complementing traditional clinical trial data. By capturing long-term patient outcomes from everyday clinical practice, RWE provides a more comprehensive view of how treatments perform across diverse populations. This shift toward value-based healthcare, where patient outcomes are prioritized over the volume of treatments, is driving the increased adoption of RWE in clinical research.
At the upcoming 7th Annual MarketsandMarkets Real-World Evidence and Life Science Analytics Conference, Aditya Tallapragada, President of AKT Health Inc., will lead a key session titled “Technological Innovations Driving Decentralized Clinical Trials and Real-World Evidence.” Scheduled for October 17, 2024, from 15:45 to 16:15, in Boston, MA, the session promises to dive deep into the intersection of technology and clinical research.
Tallapragada’s presentation will explore how emerging technologies such as AI-driven platforms, blockchain for data integrity, and advanced analytics are driving the decentralization of clinical trials. These technologies not only improve data quality but also enhance the patient experience, creating a more accessible, reliable, and scalable model for future clinical studies. He will also discuss the potential of wearable devices to revolutionize patient monitoring, offering a seamless and continuous flow of data without the need for in-person visits.
As DCTs become more prevalent and RWE continues to grow in importance, the future of clinical research looks set to embrace both technological and patient-centered innovations. The integration of these models ensures that clinical trials are not only more efficient but also more reflective of the diverse real-world patient experiences.
“As we move toward a future where clinical research reflects real-world patient experiences, we must harness both technological and human-centered innovations. By embracing decentralized models and real-world evidence, we not only improve efficiency but ensure treatments cater to the unique needs of patients across diverse communities globally. These innovations are driving a new era of clinical research, one that is both patient-centric and data-driven.” — Aditya Tallapragada, President of AKT Health Inc.”
Tallapragada’s session will offer a comprehensive overview of the ways in which Real-World Evidence and Decentralized Clinical Trials are set to redefine the future of clinical research. Attendees can expect to gain critical insights into the transformative role of technology in enabling more agile, patient-centric studies and learn how the convergence of RWE and DCTs can improve drug development and healthcare outcomes.
Don’t miss the opportunity to engage with these groundbreaking trends at the MarketsandMarkets Real-World Evidence and Life Science Analytics Conference on October 17, 2024. Join industry leaders and experts as they explore the next frontier of clinical research.
About AKT Health:
AKT Health is a strategic consulting firm focused on the healthcare and life sciences industries, offering tailored solutions that drive innovation and success. As a trusted partner to pharmaceutical companies, medical institutions, and life science organizations, we help achieve sustainable growth, regulatory compliance, and market leadership through long-term strategic planning and expert execution. Our services include medical affairs and regulatory compliance support, commercial strategy for marketing and sales, clinical operations assistance for clinical trials, and advanced technology solutions such as Remote Patient Monitoring (RPM) and Decentralized Clinical Trials (DCT) to address complex healthcare challenges.
Hema Dubey
AKTHealth
email us here
Visit us on social media:
Facebook
X
LinkedIn
Instagram
YouTube
Other
AKT Health
Distribution channels: Business & Economy, Conferences & Trade Fairs, Healthcare & Pharmaceuticals Industry, IT Industry, Technology
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release