Erythropoietin (EPO) Drugs Market Forecast To Surge USD 12.5 Billion By 2033, Driven By 5.20% CAGR
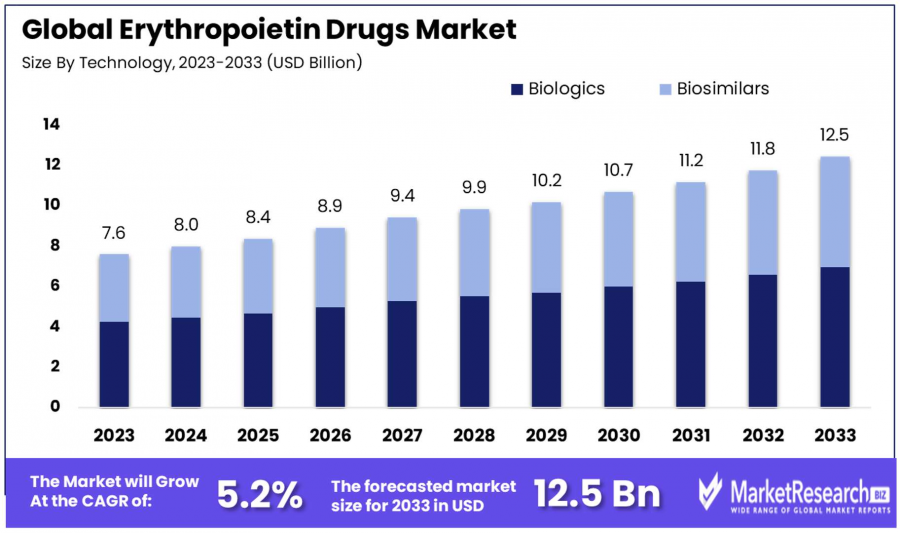
The global Erythropoietin (EPO) Drugs Market is set for significant expansion, projected to reach USD 12.5 billion by 2033 from USD 7.6 billion in 2023
NEW YORK, NY, UNITED STATES, February 7, 2025 /EINPresswire.com/ -- Report Overview
The global Erythropoietin (EPO) Drugs Market is set for significant expansion, projected to reach USD 12.5 billion by 2033 from USD 7.6 billion in 2023, with a compound annual growth rate (CAGR) of 5.20% during the 2024-2033 forecast period.
The Erythropoietin (EPO) drugs market is expanding rapidly, driven by the increasing prevalence of chronic kidney disease (CKD), cancer-related anemia, and other blood disorders. EPO drugs are used to stimulate red blood cell production, helping patients manage anemia effectively.
With rising demand for biologics and biosimilars, pharmaceutical companies are investing in next-generation EPO formulations to improve efficacy and reduce side effects. Advanced EPO variants, such as darbepoetin alfa and epoetin beta, offer extended half-life, reducing dosing frequency for patients. Regulatory agencies, including the FDA and EMA, are approving new EPO biosimilars to enhance market accessibility and affordability. Governments and healthcare organizations are also increasing awareness and funding programs to improve treatment options for anemia patients.
Key industry players are focusing on innovative drug delivery mechanisms, including long-acting injectables and gene therapy-based EPO alternatives, to improve patient compliance and outcomes. With rising investments in R&D and biosimilar development, the EPO drugs market is set for significant growth in the coming years.
Unlock Competitive Advantages With Our PDF Sample Report @ https://marketresearch.biz/report/erythropoietin-epo-drugs-market/request-sample/
Key Takeaways
- Market Value: The Erythropoietin Drugs Market is projected to grow from USD 7.6 billion in 2023 to USD 12.5 billion by 2033, at a CAGR of 5.20% during 2024-2033.
- By Type: Biologics dominate with a 56% market share due to proven efficacy and safety, while biosimilars are gaining traction for cost-effectiveness and broader treatment accessibility.
- By Product: Erythropoietin holds 76.9% of the market, widely used for anemia treatment, while darbepoetin-alfa remains a strong competitor due to longer dosing intervals.
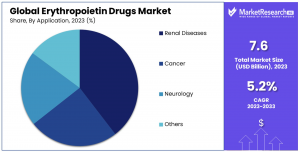
- By Application: Renal diseases lead with a 40.5% market share, followed by cancer and neurology, highlighting EPO’s diverse therapeutic applications.
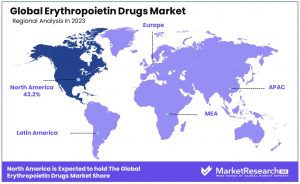
- Regional Analysis: North America leads with 43.2% market share, driven by advanced healthcare infrastructure, followed by Europe, where biosimilar adoption and healthcare accessibility contribute to growth.
- Key Players: Leading companies include Amgen, Johnson & Johnson, Roche Ltd., Pfizer, Biocon, and Teva Pharmaceutical, among others.
- Analyst Viewpoint: Market growth is fueled by the rising burden of chronic diseases, increasing biosimilar adoption, and demand for cost-effective therapies.
- Growth Opportunities: Expansion into emerging markets, biosimilar advancements, and exploration of new therapeutic applications are key growth drivers in the forecast period.
How Artificial Intelligence (AI) is Transforming the Erythropoietin (EPO) Drugs Market ?
• AI in Drug Discovery & Development: AI accelerates EPO drug research by analyzing large datasets to identify new formulations and biosimilar alternatives. Machine learning models predict drug interactions and optimize protein modifications, leading to improved stability and efficacy of EPO therapies.
• AI-Powered Manufacturing & Quality Control: AI enhances biologic drug manufacturing by ensuring precision in protein synthesis, optimizing production efficiency, and reducing waste. AI-based predictive maintenance prevents equipment failures in EPO drug production, improving batch consistency and regulatory compliance.
• AI for Personalized Treatment Plans: AI-driven algorithms analyze patient medical records and biomarkers to tailor EPO drug dosages. This ensures optimized erythropoietin therapy for patients with chronic kidney disease (CKD), cancer-induced anemia, and neurological disorders.
• AI in Predictive Analytics for Patient Outcomes: AI tools monitor patient responses to EPO drugs, predicting risk factors like thrombotic events and resistance to treatment. This allows physicians to adjust dosages and treatment regimens in real time, improving safety and effectiveness.
• Future Outlook: With AI’s expanding role in drug discovery, personalized medicine, and digital health monitoring, the EPO drugs market is set to benefit from faster innovation, cost reductions, and enhanced treatment precision. As AI technologies evolve, biosimilar development and advanced EPO formulations will continue driving market growth.
Segmentation Analysis
The market segmentation is primarily categorized by type, product, and application. Biologics hold a dominant position in the "By Type" segment, accounting for a 56% market share, favored for their proven efficacy and longstanding safety. Biosimilars are emerging, providing cost-efficient alternatives. In the "By Product" segment, Erythropoietin leads with a 76.9% share due to its broad application in anemia management across various medical conditions. The "By Application" segment highlights Renal Diseases as the leading sub-segment with 40.5% share, driven by the high prevalence of CKD and ESRD globally. Cancer and Neurology applications also demonstrate significant market presence due to the need to manage anemia from treatment regimens like chemotherapy, underscoring the versatility and expanding role of EPO drugs.
Market Segments
By Type
•Biologics
•Biosimilars
By Product
•Erythropoietin
•Darbepoetin-alfa
By Application
•Renal Diseases
•Cancer
•Neurology
•Others
Buy This Premium Research Report: https://marketresearch.biz/purchase-report/?report_id=2702
Market Dynamics
• Driver: The increasing prevalence of chronic diseases, such as chronic kidney disease (CKD) and cancer, significantly drives the demand for erythropoietin (EPO) drugs. EPO drugs are essential in managing anemia associated with these conditions by stimulating red blood cell production. The World Health Organization (WHO) highlights the rising global burden of non-communicable diseases, emphasizing the need for effective treatments like EPO therapies.
• Trend: A notable trend in the EPO drugs market is the shift towards biosimilars to enhance treatment accessibility and affordability. Biosimilars offer comparable efficacy and safety profiles to original biologics but at reduced costs. The WHO supports the adoption of biosimilars, recognizing their potential to improve access to essential medicines and promote sustainable healthcare systems.
• Restraint: Stringent regulatory requirements for the approval of EPO drugs pose a significant restraint in the market. The complex manufacturing processes and the need to demonstrate biosimilarity in terms of efficacy, safety, and quality necessitate extensive clinical trials and data, leading to increased development costs and time. The European Medicines Agency (EMA) outlines comprehensive guidelines for biosimilar approval, reflecting the rigorous standards that manufacturers must meet.
• Opportunity: Expanding EPO drug applications beyond traditional indications presents a significant opportunity in the market. Research into novel therapeutic areas, such as neuroprotection and wound healing, where EPO's erythropoietic and tissue-protective properties could be beneficial, is ongoing. The WHO encourages innovation in drug development to address unmet medical needs, highlighting the potential for EPO drugs in emerging therapeutic areas.
Regional Analysis
The Erythropoietin Drugs Market features several leading players with strong research and development capabilities. Amgen, Johnson & Johnson, and Roche Ltd. are at the forefront, leveraging their market presence and R&D resources to maintain significant shares through innovative drug formulations and patient-centric approaches. Pfizer, Biocon, and Teva Pharmaceutical are strategically positioned by expanding their product portfolios. Companies like LG Life Sciences and Thermo Fisher Scientific contribute with their expertise in biopharmaceuticals, enhancing market growth. Additionally, Sandoz and Dr. Reddy's Laboratories focus on biosimilars, driving competition and expanding treatment access.
Competitive Landscape
The Erythropoietin Drugs Market features several leading players with strong research and development capabilities. Amgen, Johnson & Johnson, and Roche Ltd. are at the forefront, leveraging their market presence and R&D resources to maintain significant shares through innovative drug formulations and patient-centric approaches. Pfizer, Biocon, and Teva Pharmaceutical are strategically positioned by expanding their product portfolios. Companies like LG Life Sciences and Thermo Fisher Scientific contribute with their expertise in biopharmaceuticals, enhancing market growth. Additionally, Sandoz and Dr. Reddy's Laboratories focus on biosimilars, driving competition and expanding treatment access.
Top Key Players
•Amgen Inc.
•Johnson & Johnson
•F. Hoffmann-La Roche Ltd.
•Pfizer
•Biocon
•Teva Pharmaceutical Industries Ltd.
•Kyowa Kirin
•LG Life Sciences Ltd.
•Thermo Fisher Scientific
•Sandoz International GmbH
•3SBio Inc.
•Probiomed
•Intas Pharmaceuticals Ltd.
•Dr. Reddy's Laboratories
•Celltrion Inc.
•Hospira Inc.
•Watson Pharmaceuticals
•GlycoMimetics Inc.
•Akebia Therapeutics
Emerging Trends in Erythropoietin (EPO) Drugs
• Development of Biosimilar EPO Drugs: As patents for original EPO drugs expire, biosimilar versions are being developed to provide more affordable treatment options. These biosimilars are designed to be as effective and safe as the original biologics, potentially reducing healthcare costs and increasing patient access.
• Exploration of Non-Hematopoietic Uses: Research is expanding into EPO's protective effects beyond red blood cell production. Studies suggest potential benefits in treating conditions like brain injuries and other organ diseases, indicating a broader therapeutic application for EPO drugs.
Use Cases of Erythropoietin (EPO) Drugs
• Anemia Management in Chronic Kidney Disease (CKD): EPO drugs are commonly used to treat anemia in CKD patients. By stimulating red blood cell production, they reduce the need for blood transfusions and improve patients' quality of life. For instance, epoetin alfa has been shown to increase hemoglobin levels by more than 3 g/dL in CKD patients.
• Anemia Associated with Cancer Chemotherapy: Cancer patients undergoing chemotherapy often develop anemia due to myelosuppression. EPO drugs can effectively counteract this by accelerating the recovery of the erythroid compartment, reducing fatigue, and enhancing patients' ability to continue treatment.
• Potential Neuroprotective Applications: Beyond anemia treatment, EPO has been investigated for its neuroprotective properties. Research indicates that EPO may offer protective benefits in various neurological conditions, suggesting potential therapeutic applications in the nervous system.
Lawrence John
Prudour
+91 91308 55334
email us here
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release