FUJIFILM Healthcare Europe announces revolutionary Detective Flow Imaging (DFI) technology compatibility with mainstream EG-580UT endoscope
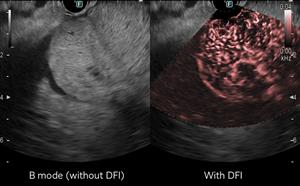
/EIN News/ -- RATINGEN, Germany, July 16, 2024 (GLOBE NEWSWIRE) -- FUJIFILM Healthcare Europe GmbH is pleased to announce that the Detective Flow Imaging feature (DFI) of the premium ARIETTA 850 FF Endo ultrasound system is now available via update with our mainstream endoscope, the EG-580UT. This update allows even more physicians to experience clarity in motion during diagnostic and therapeutic procedures.
The revolutionary DFI technology in the ARIETTA 850 FF Endo system is now compatible with our mainstream EG-580UT ultrasound endoscope. This compatibility allows physicians to experience the benefits of DFI technology firsthand. The EG-580UT endoscope features a small bending radius and short rigid section, enabling easy access to targeted areas. Its wide puncture range enables Fine Needle Aspiration Biopsy (FNA) from various positions, providing broader accessibility. The 40° front oblique view and 140° endoscopic field gives an optimal luminal view during insertion, and its powerful 150° up-angulation makes it suitable for both observation and therapeutic procedures.
A recent study highlighted the advantage of DFI technology, stating, "DFI-EUS allows the evaluation of vascularization patterns of pancreatic tumors similar to those obtained by CEH-EUS, with a similar diagnostic yield, but without requiring the use of intravenous contrasts."1
The availability of DFI technology with the mainstream EG-580UT endoscope allows physicians to benefit from the clarity and advanced diagnostic capabilities of DFI during endoscopic procedures. This update represents our commitment to continuously improving patient outcomes and enhancing the capabilities of our endoscopic ultrasound systems.
For more information about the ARIETTA 850 FF Endo ultrasound system and the EG-580UT endoscope, please visit www.fujifilm-endoscopy.com.
____________________
1 J. Iglesias-Garcia et al. “Endoscopic ultrasound (EUS)-guided detective flow imaging (DFI). A new advanced imaging technique for the differential diagnosis of pancreatic solid tumors” Endoscopy 2024; 56, https://www.thieme-connect.de/products/ejournals/abstract/10.1055/s-0044-1783535
About FUJIFILM Healthcare Europe
After the acquisition of Hitachi’s diagnostic imaging business in 2021, the European headquarters of FUJIFILM’s Medical Systems Business Unit became FUJIFILM Healthcare Europe in 2023 to maximise the value of the combined product portfolio. Alongside the formation of the new strategic regional headquarter, various local Fujifilm subsidiaries followed the way of forming dedicated Healthcare entities with the aim of bringing together knowledge, passion and inspiration to create increased value for customers. More than 1500 employees strive to support Healthcare Professionals in improving patients’ quality of life by developing the most comprehensive and innovative med-tech solutions portfolio derived from Fujifilm’s core competence in medical imaging. For more information, please visit: fujifilm.com.
Contact information
Stefan Bachmeier
Marketing Manager Europe Endoscopy Systems
FUJIFILM Healthcare Europe GmbH
Mail: stefan.bachmeier@fujifilm.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/024c5be9-367a-4e80-9163-9ecd3904dcca
https://www.globenewswire.com/NewsRoom/AttachmentNg/47a20d2f-53ff-4e53-8752-00ca04d9ba54

EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.